A new treatment for brain tumours
The story so far:
I have a glioma. It’s probably an astrocytoma but my biopsy was inconclusive. It’s probably low grade but it might be grade 3. I have had no treatment.
There have been no significant improvements in glioma care since PCV in the 1960s and TMZ in 1999. Radiotherapy has got a little bit better and surgery has improved incrementally but there have been no drug breakthroughs in over 20 years. Gliomas are stuck with PCV & TMZ which are essentially chemo while every other kind of cancer is getting breakthroughs in immunotherapy and targeted therapy.
Targeted therapy typically aims at a specific receptor or enzyme or protein and causes a cell to change its behaviour while chemo typically just kills everything and hopes that the cancer cells die faster than the healthy cells. We’ve been dreaming of a targeted therapy that can cross the blood-brain barrier AND target a receptor on glioma cells since forever.
There are three main kinds of glioma: oligodendroglioma, astrocytoma and glioblastoma. None of them are very nice but glioblastoma is particularly horrid. Almost all clinical trials for gliomas are for glioblastoma.
https://www.smartpatients.com/trials?search[keywords]=brain+tumor
I know of only two interesting trials for low-grade gliomas. One is for vorasidenib, which is a targeted therapy.
https://www.smartpatients.com/trials/NCT04164901
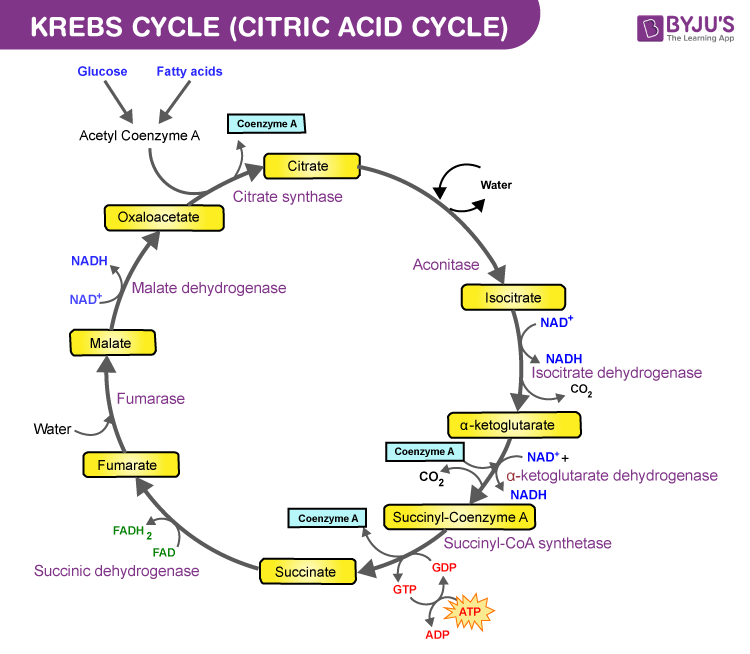
Vorasidenib targets the enzyme IDH.
IDH (Isocitrate dehydrogenase) is an enzyme that is involved in the cycle that turns nutrients into energy. In one step of the cycle, IDH is supposed to generate alpha-KG (alpha-ketoglutarate) but, if you have a particular mutation (IDH1-mt) in your IDH1 gene, it generates 2-HG (2-hydroxyglutarate) instead. Besides messing with your metabolism, 2-HG is thought to mess with your DNA, causing lots of knock-on mutations which lead to tumours.
IDH1-mt is diagnostic for two of the three main kinds of glioma: oligodendroglioma and astrocytoma (but not glioblastoma) and vorasidenib has been shown to improve survival of low-grade glioma patients to an extent that is frankly miraculous. So miraculous that they terminated the trial and gave the drug to all the participants.
I hope I survive long enough for it to be approved in the UK.